Abstract
Currently, all FDA approved additive solutions for blood storage (AS-1, AS-3, AS-5, etc.) contain glucose in concentrations of at least 45 mM, 5 times greater than diabetic blood glucose levels. We hypothesize that hyperglycemic red blood cell (RBC) storage conditions contribute to the development of irreversible physiological changes, or "storage lesions", typically measured in long-term RBC storage. These storage lesions include increased hemolysis, reduced ATP release, increased oxidative stress, and many other physiological damages We have reported normoglycemic blood storage solutions, similar to the citrate-phosphate-dextrose (CPD) solution and AS-1 additive solution, modified to contain physiological levels of glucose, termed CPD-N, and AS-1N, respectively. To maintain normoglycemic levels, the stored RBCs must be periodically re-fed with a concentrated glucose solution. Our previous reports utilized in-house prepared collection bags and manual glucose feeding, which resulted in increased ATP release, increased deformability, decreased sorbitol levels, and increased nitric oxide (NO) production by endothelial cells exposed to the RBCs. However, these methods did not utilize commercial blood storage bags and also employed a manual feeding regimen that is not feasible in current blood banking practices.
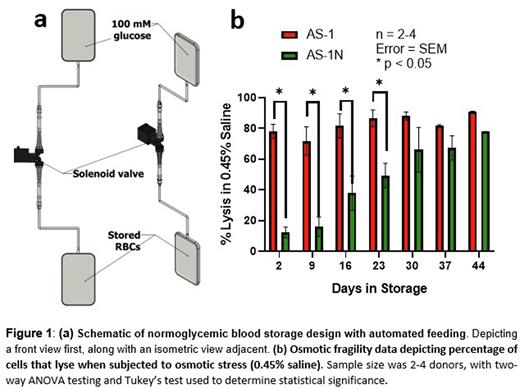
Here, we present a novel, fully automated glucose feeding system utilizing the normoglycemic additive solutions, CPD-N and AS-1N, with stored RBCs in commercial blood collection bags. Typical storage lesion indicators were monitored throughout storage duration, including ATP release, sorbitol levels, hemolysis, and osmotic fragility. The design of the automated feeding system included a solenoid valve that connects an IV bag of a concentrated glucose solution to a stored RBC bag (Figure 1a). The solenoid valve was controlled by an Arduino Uno microcontroller programmed to open the valve at 3-day intervals and dispense precise volumes of glucose solution into the stored RBC product. Successful calibration of the valve revealed a lower dispensing volume limit of approximately 200 μL, and an average relative error of less than 7% within the range of valve opening times tested. The valve system was able to successfully maintain physiological glucose concentrations in stored RBCs in the range of 4-6 mM for 44 days without intervention.
Measurements of ATP release from the stored blood revealed that RBCs stored in AS-1N maintained initial levels of extracellular ATP (approximately 50 nM) throughout the 44 day storage period, while RBCs stored in AS-1 had approximately 50% less ATP release, which declined during storage. Additionally, cells stored in AS-1N displayed significantly greater resistance to osmotic stress through day 23 of storage, with up to a 65% decrease in cell lysis when subject to osmotic changes (Figure 1b). Although there was no significant difference in the amount of hemolysis between AS-1 and AS-1N stored blood, both storage approaches resulted in less than 1% cell lysis throughout the storage duration, indicating that the glucose feeding regimen had no harmful effect on levels of hemolysis. Sorbitol was measured as an indicator of oxidative stress in both AS-1 and AS-1N stored blood and showed that RBCs stored in AS-1N led to decreased sorbitol levels in comparison to AS-1, and maintained lower levels throughout storage, suggesting decreased oxidative damage in comparison to traditional AS-1 RBC storage.
These results confirm previous findings that normoglycemic storage solutions decrease levels of storage lesion markers. Additionally, the novel automated feeding system allows for this technology to now be realistically translated to current blood banking procedures and practices.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal